HCP and Patient Targeted Risk Minimization Measures (RMMs)
*Formerly refered to as "Risk Management Tools" or RMT, we have revised to the term "measures" and RMM to align with Health Canada terminology*
Background:
PAAB has received several questions and submissions for HCP and patient targeted Risk Minimization Measures (RMMs). The present document is intended to guide industry in creation of compliant RMMs. Although the principles discussed in this guidance have been reviewed by Health Canada, this document may be superseded by future Health Canada guidance.
Note that the terms risk minimization tool, risk management tool, risk minimization measure, and risk management measure are often used interchangeably.
Risk Management Measures are documents which are part of a Health Canada mandated or Global mandated risk management plan or program. This means that some RMMs are mandated and approved by Health Canada while others are Global initiatives (which are not reviewed by Health Canada). The latter is more common than the former.
The purpose of RMMs is to convey important identified risks, important potential risks, and missing information about a manufacturer’s products. They do not contain claims of benefit as these measures are created solely with the aim of minimizing / managing / mitigating risk. Although distribution of such measures need not be in response to unsolicited requests, they are NOT intended or destined for promotional activities/uses and may not be used in such ways unless they undergo standard PAAB approval.
The PAAB encourages the sponsors to keep Health Canada aware of involvement in risk minimization activities.
How does PAAB approach RMM assessment?
Consider the following factors:
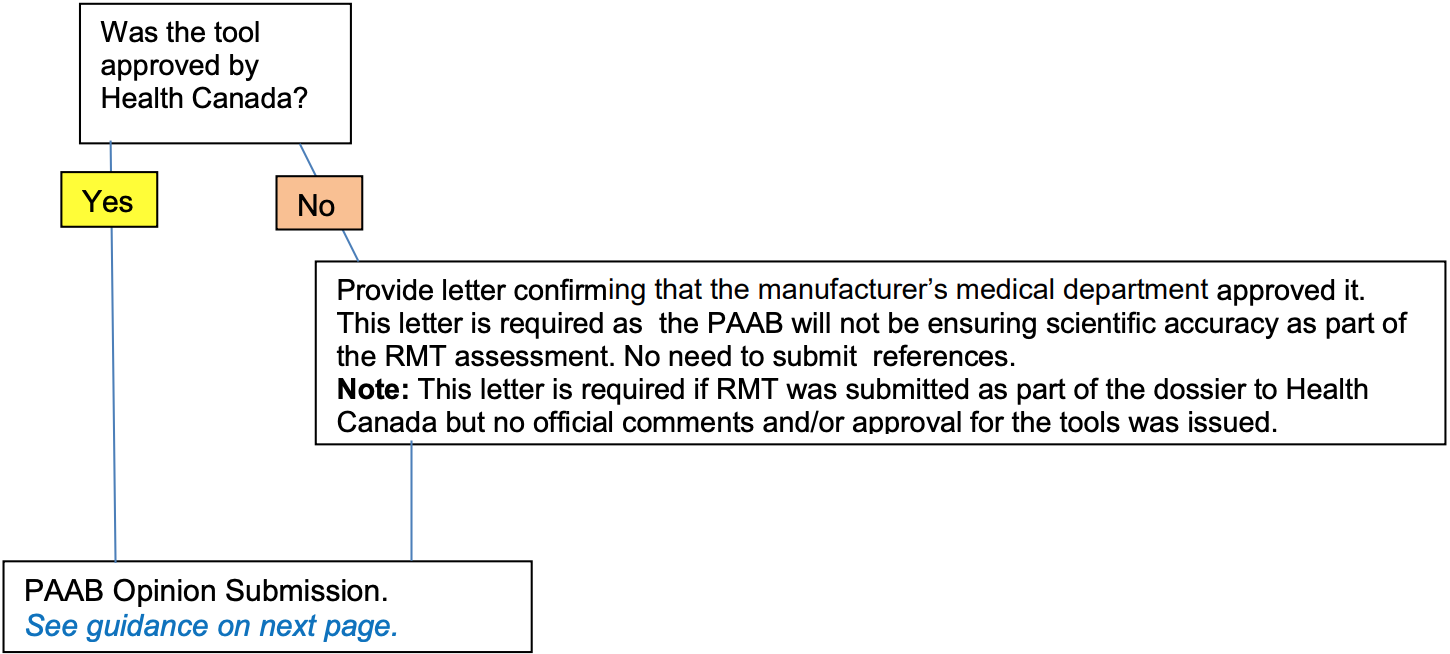
| Guidance for PAAB Opinion Submissions Relating to RMMs (i.e. “yes” is the response to at least one question in figure 1) |
- As these risk management measures are not created with the intent to promote products, PAAB’s role is to ensure that the materials are non-promotional or to inform manufacturers of the revisions required to render the measure non-promotional (such that it does not directly or indirectly contravene the legislative and regulatory advertising provisions of the Food and Drugs Act and Regulations ).
- PAAB’s review takes the form of a written opinion based on direction from Health Canada and consideration of the general principles in the Health Canada policy document “The Distinction Between Advertising and Other Activities”. See the fee schedule on the PAAB website for costs relating to the opinion service.
| Specific Guidance for PAAB Opinion Submissions Relating to RMMs | |
|---|---|
| Manufacturer letter |
A letter from the manufacturer confirming that Health Canada approved the RMM.
OR (when not approved by Health Canada)
A signed letter from the manufacturer’s medical department (or equivalent) confirming that the entire measure has been internally reviewed and approved for scientific accuracy and consistency with the product monograph. |
| Tonality
|
The tone must be one of caution throughout the document. The purpose of any message within such piece is to minimize, manage, and inform about risk. Appropriate dosing, for example, is one way to manage risk. |
| Statements of benefit |
There may be no direct or implied content about product benefits (even if scientifically accurate) and no product claims (whether comparative or non-comparative). See additional considerations for HCP targeted measures below. |
| Product Branding Elements |
Generally, the product logo and colour scheme may be employed for RMMs pertaining to the corresponding particular product. This is not a requirement. See exception below relating to off-label content. |
| Off-label |
Only uses authorized in Canada should be discussed unless distribution of the measure will be limited to unsolicited requests. PAAB will consult with Health Canada if discussion of off-label use is critical to appropriate risk management. |
| Title
|
The document name should not imply that Health Canada has approved the document. In the past, Health Canada requested that the term “Safety monograph” not be used. The combination of ‘Safety’ with ‘monograph’ may suggest that Health Canada has provided full approval of the measure. |
| Disclaimer
|
The first surface (e.g. exterior front cover) must carry a statement along the lines of “This material was developed by [manufacturer name], as part of the risk minimization plan for [product name]. This material is not intended for promotional use”. If this is not a product measure, the segment ‘for [product name]’ must not be used. |
| PAAB logo |
The PAAB opinion assessment is generally limited to confirming that the measure is non-promotional and that the criteria in this document are met (e.g. the content was not assessed by PAAB for scientific/clinical accuracy). The measure, therefore, should not contain the PAAB logo. |
| References | Given the nature of the PAAB assessment described above, references need not be included in the submission. Exception: The relevant regulatory references such as the most recent product monograph and NOC letter are required. |
| Modifications |
It is important for manufacturers to update risk management measures as new information becomes available. Modified RMMs should be resubmitted to the PAAB for assessment. |
| Renewals |
Although a no objection letter will be provided upon completion of the review, it will not include an expiration date. Renewals are therefore not required for RMMs. See “modifications” in the section above. |
| Context of use |
Although RMMs can be promoted and distributed by representatives, they must not be used as detailing aids during a sales call unless full review and approval from PAAB is obtained (i.e. rather than an opinion submission). On-label product branded RMMs can be distributed with product branded APS (see provisions below relating to off-label content below). |
If the measure contains any off-label content (only after consultation with Health Canada), there may be no :
- use of the product logo or product branding colours
- distribution in promotional contexts (e.g. sales reps)
- distribution or housing alongside product branded (and/or promotional) materials
- mention of the measure in branded and/or promotional materials
| Considerations specific to the target audience for PAAB Opinion Submissions Relating to RMMs |
Patient
measures intended for patients should be comprised of non-promotional discussion of risks and mitigation strategies that are relevant to a patient on that particular drug. i.e. documented or potential risks for the prescribed drug and/or that product’s class in general . Other prescription healthcare products/classes may not be mentioned. It is acceptable to use language along the lines of “As a risk of X has been observed for some products used in the treatment of this condition, you should keep an eye out for X and inform your doctor if it occurs”.
EXCEPTION relating to mention of other specific products: other prescription healthcare products can be discussed to the extent that they are discussed in part III of the prescribed drug’s product monograph and/or its indication. Additionally, if the measure is intended to be distributed specifically to patients taking two products concomitantly, this should be conveyed prominently on the measure’s front cover, along with identifying the specific products.
HCP
measures intended for HCPs should be comprised of non-promotional discussion of risks and mitigation strategies relating to the sponsor’s product/class OR risk messages relating to other products framed as potential risks of the sponsor’s product. Presentations conveying that the manufacturer’s product does not have a particular risk (or poses less risk than other products/classes/categories) are not acceptable.
Manufacturers should exercise caution when summarizing study results as this has the potential to provide an incomplete (and therefore inaccurate) picture of the available evidence and may introduce bias, which could render the material subject to all advertising regulations. The sponsor’s medical department must ensure the presentation does not omit important data relevant to the sponsor product’s risk.
FAQ:
-
What if the manufacturer plans to:
- Instruct/train drug representative to detail from the RMM during a sales call in order to make sure HCPs consume this important content?
- Include product claims and statements of benefits in order to establish balance in the measure?
The piece would simply be subject to all PAAB code provisions relating to APS and to standard review times.
- Can PAAB approved APS mention on-label RMMs?
Yes. Product branded APS can mention on-label product branded RMMs. ( see provisions below relating to off-label content above ).
- Can on-label RMMs appear on websites along with with PAAB approved APS (e.g. on a gated website).
Yes. Product branded APS need not be stored in separate silos from on-label product branded RMMs . The manufacturer should ensure to clearly distinguish between RMMs and PAAB approved APS (i.e. PAAB approved APS contain the PAAB logo while RMMs contain the disclaimer discussed above) .
-
Will PAAB provide an approval number and approval period?
As no PAAB logo should appear on the piece, no approval number or approval period will be provided. This means there is no need to renew the piece annually.
The final PAAB letter will read “The PAAB has no objection to the attached piece. This piece does not require renewal. Note that future modifications made to the piece should be resubmitted to the PAAB for assessment.”
-
What are the timelines for this assessment?
The standard timelines for an opinion (4 business days for first response, 3 business days for revisions).
-
What are the review fees?
Same fee structure as APS on our fee schedule: http://www.paab.ca/fee-schedule-services.htm

