Section 4.2.3 of the PAAB Code states:
Reporting clinical trial results in relative or proportional terms may lead to misinterpretation of the true benefit and degree of a treatment effect. APS which present results using these methods of reporting, namely relative risk (RR) or relative risk reduction (RRR), must also include an indication of the absolute treatment effect. This can be presented as absolute risk reduction (ARR), number needed to treat (NNT) and/or the actual comparative clinical results or rates. The overall presentation should reflect the true magnitude of benefit and not magnify the clinical effect. Undue emphasis on treatment effects in relative terms, by means of graphic presentation or differences in type size, is not acceptable.
This guidance document clarifies the PAAB's interpretation of "undue emphasis" in order to support consistent application of code section 4.2.3 in drug adverting.
Basic factors when assessing emphasis:
- Font – Absolute results should be presented with similar prominence to the relative risk claim. As a general guidance, the size of absolute results should be no less than 75% of the size of the relative risk claim (i.e. x-height, cap height). Additional factors, such as font type, font weight, height of ascenders/descenders, letterspace/kerning and line spacing/leading will also be considered (please see Guidance on Font Sizing and Selection for Indication Disclosure and Fair Balance for more information).
- Position – The absolute results should form an integral part of the relative risk claim.
Additional factors, including but not limited to colour, contrast and graphic components, will be considered when assessing emphasis.
Previously accepted material will be reassessed and revisions may be required.
The following examples are intended to help in the development of advertising materials that present claims in relative terms.
Example 1
Unacceptable
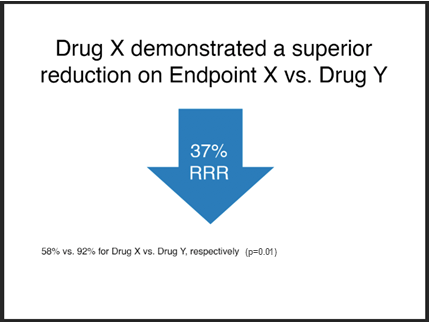
Notes:
- The font size of the absolute results, “58% vs. 92%...”, is less than 75% that of the relative risk claim, “37% RRR”.
- The absolute results have not been integrated into the relative risk claim.
Acceptable
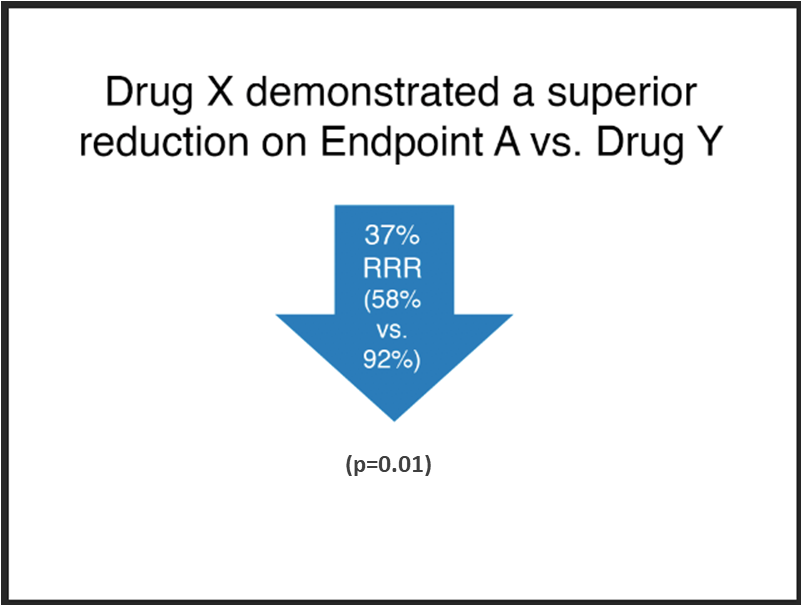
Notes:
- The font size of the absolute results, “58% vs. 92%”, is at least 75% that of the relative risk claim, “37% RRR” (it is at 90% in this example).
- The absolute results have been integrated into the relative risk claim.
Example 2
Unacceptable
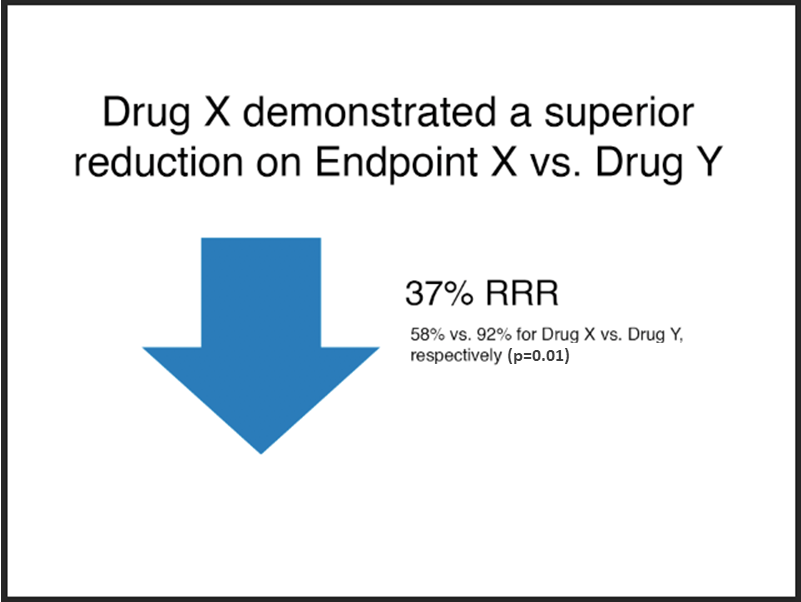
Note:
- The font size of the absolute results, “58% vs. 92%...”, is less than 75% that of the relative risk claim, “37% RRR”.
Acceptable
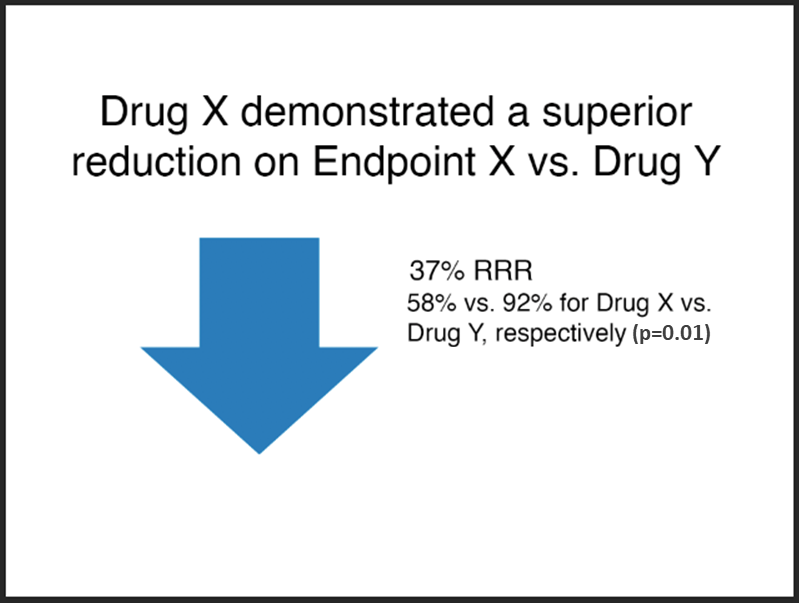
Note:
- The font size of the absolute results, “58% vs. 92%...”, is at least 75% that of the relative risk claim, “37% RRR” (it is at 90% in this example).
Example 3
Unacceptable
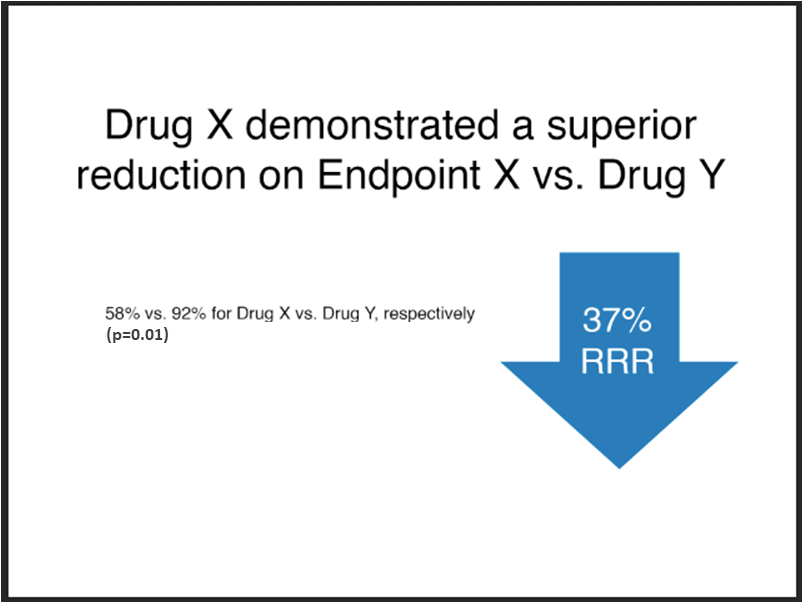
Note:
- The font size of the absolute results, “58% vs. 92%...”, is less than 75% that of the relative risk claim, “37% RRR”.
- The absolute results have not been integrated into the relative risk claim.
Acceptable
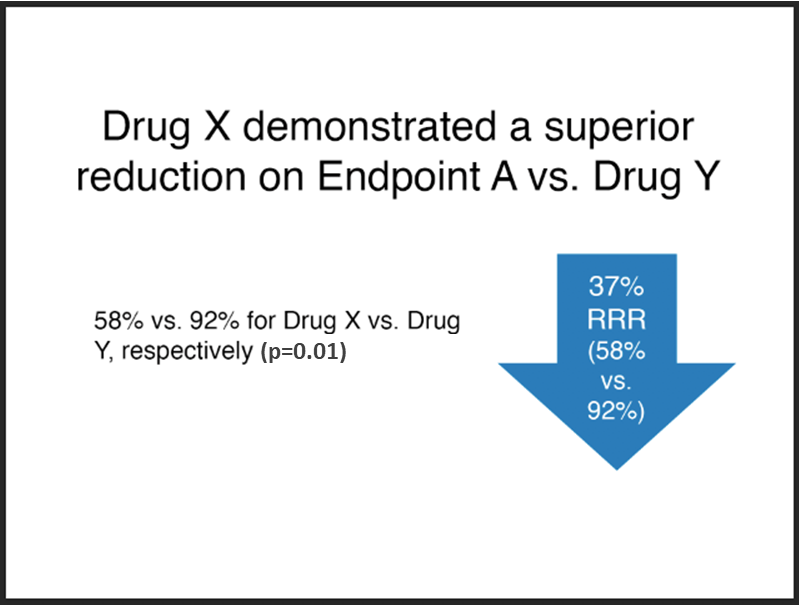
Note:
- The font size of the absolute results, “58% vs. 92%...”, is at least 75% that of the relative risk claim, “37% RRR” (it is at 90% in this example).
- The absolute results have been integrated into the relative risk claim.

