PAAB has seen a number of real-world evidence that have a similar design. This advisory serves as a guide to our approach to this type of study and is intended as a supplement to the Guidance on Real-World Evidence/Data.
1. What type of RWE is the focus of this advisory?
These are single-arm studies evaluating a product in patients who were previously treated with other products. The studies can be prospective or retrospective in design. A concern with this study design is the comparative implications between the study drug and the previous treatments.
The design of these studies should be disclosed in the APS for transparency and to set the context for the presentation.
To illustrate, please see the graphic below.
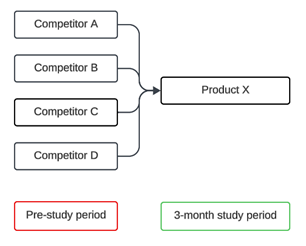
For the purposes of the examples below, this hypothetical study was designed to assess the effectiveness and safety of the sponsor’s drug, Product X, during a 3-month study period in patients who had received other treatments, Competitors A, B, C and D, for the condition previously; Competitors C and D belong to Drug Class Y. The primary endpoint was a measure of effectiveness: mean change in IOP from baseline at 3 months. Rates of ocular hyperemia, an adverse event, was a secondary endpoint.
2. Effectiveness messages
Effectiveness endpoints evaluate outcomes relating to the medical condition that the product is indicated for. These can include quality of life measurements.
Message example 1a: “In previously treated patients, the demonstrated mean change in IOP from baseline at 3 months with Product X was 5.1 mmHg”
If the study was designed to reflect the general marketplace and was not designed to exclude specific drugs or classes of drugs, such a message can be acceptable.
NOTE: Referring to the hypothetical study, if Competitors A, B, C and D reflect the general marketplace, the message may be acceptable. If through chance, and not by design, the study did not include patients who were previously treated with Competitor A, the message may also be acceptable. This is a natural exclusion rather than an exclusion by design.
If the study was not designed to reflect the complete marketplace and, instead, targeted patients who had been previously treated with a select group/class of drugs (i.e., exclusions were by design), such a message would not be acceptable similar to example 2.
EXCEPTION 1: Consistency with the indication is required. If Product X is indicated to be used following specific other product(s) or product class(es), message example 1 may be acceptable based on a study that targeted patients who had been previously treated with those other therapies. The study should not be designed to exclude patients treated previously with any of these specific options.
EXCEPTION 2: There may be compelling reasons that reflect real-world clinical practice for products to be prescribed in a certain sequence. One example of this is step-up therapy laid out in authoritative consensus guidelines. In such cases, message example 1 may be acceptable even if the study targeted patients who had been previously treated with a select group/class of drugs. Sponsors should provide the necessary support for this exclusion by design.
Message example 2: “In patients previously treated with Competitor A, the demonstrated mean change in IOP from baseline at 3 months with Product X was 4.8 mmHg”
This message singles out Competitor A and creates comparative implications vs. Competitor A. Such a message is not acceptable. Comparative messages should be based on head-to-head studies in which both groups are treated in a comparable manner as per s. 1.7 of the Guidance on Real-World Evidence/Data.
EXCEPTION 1 applies to this message example if Product X was indicated in patients previously treated only with Competitor A and this subgroup analysis was preplanned.
EXCEPTION 2 applies to this message example if there is a compelling reason for Product X to be prescribed only to patients who were previously treated with Competitor A and this subgroup analysis was preplanned.
ADDITIONAL CONSIDERATION: This message example may be acceptable if the study also evaluated patients treated with Competitor A after they had been treated with Product X. These results should be included in the APS.
Message example 3: “In patients previously treated with Drug Class Y (Competitor C and Competitor D), the demonstrated change in IOP from baseline at 3 months with Product X was 5.6 mmHg”
Similar to example 2, such a message highlighting a group of drugs (Drug Class Y) is not acceptable.
EXCEPTION 1 applies to this message example if Product X was indicated in patients previously treated only with Drug Class Y and this subgroup analysis was preplanned.
EXCEPTION 2 applies to this message example if there is a compelling reason for Product X to be prescribed only to patients who were previously treated with Drug Class Y and this subgroup analysis was preplanned.
ADDITIONAL CONSIDERATION: This message example may be acceptable if the study also evaluated patients treated with Competitor C and Competitor D after they had been treated with Product X. These results should be included in the APS.
3. Safety/tolerability messages
Message example 4: “The most commonly observed adverse effects seen during the study were hyperemia (20.2%), eye irritation (8.3%) and blurred vision (5.4%)”
Such a message can be acceptable if it is generally consistent with the product’s Terms of Market Authorization.
Message example 5: “The rates of ocular hyperemia at baseline and 3 months were 21.1% and 17.5%, respectively”
Unlike effectiveness endpoints, which assess a drug's impact on an existing medical condition, side effects arise as a consequence of drug therapy. Therefore, given the study structure described above, any changes observed from the baseline to the end of the study period are implicit comparisons to the previous therapy. This message is not acceptable.

